• We process the necessary import permits.
• If we have direct contact with the CRO (Contract Research Organization), we act as the authorized importer.
What is a Research Protocol?
A research protocol is a document that outlines how a clinical study (for developing new drugs) should be conducted. It defines the objectives, design, methodology, statistical considerations, and general organization of the trial.
Main Stakeholders:
- Sponsor: The entity financing the study.
- CRO (Contract Research Organization): A company acting on behalf of the sponsor, managing the operational and logistical aspects of the study.
- Central Laboratory:Usually located in the U.S., responsible for processing and analyzing biological samples.
- Authorized Importer: Registered and authorized by COFEPRIS to import medications, medical kits, and other protocol supplies. Responsible for handling sanitary permits and customs clearance.
- Research Sites (Hospitals, Clinics, Centers): Locations where the protocol is carried out, patients are treated, and samples are collected.
Logistics in research protocols
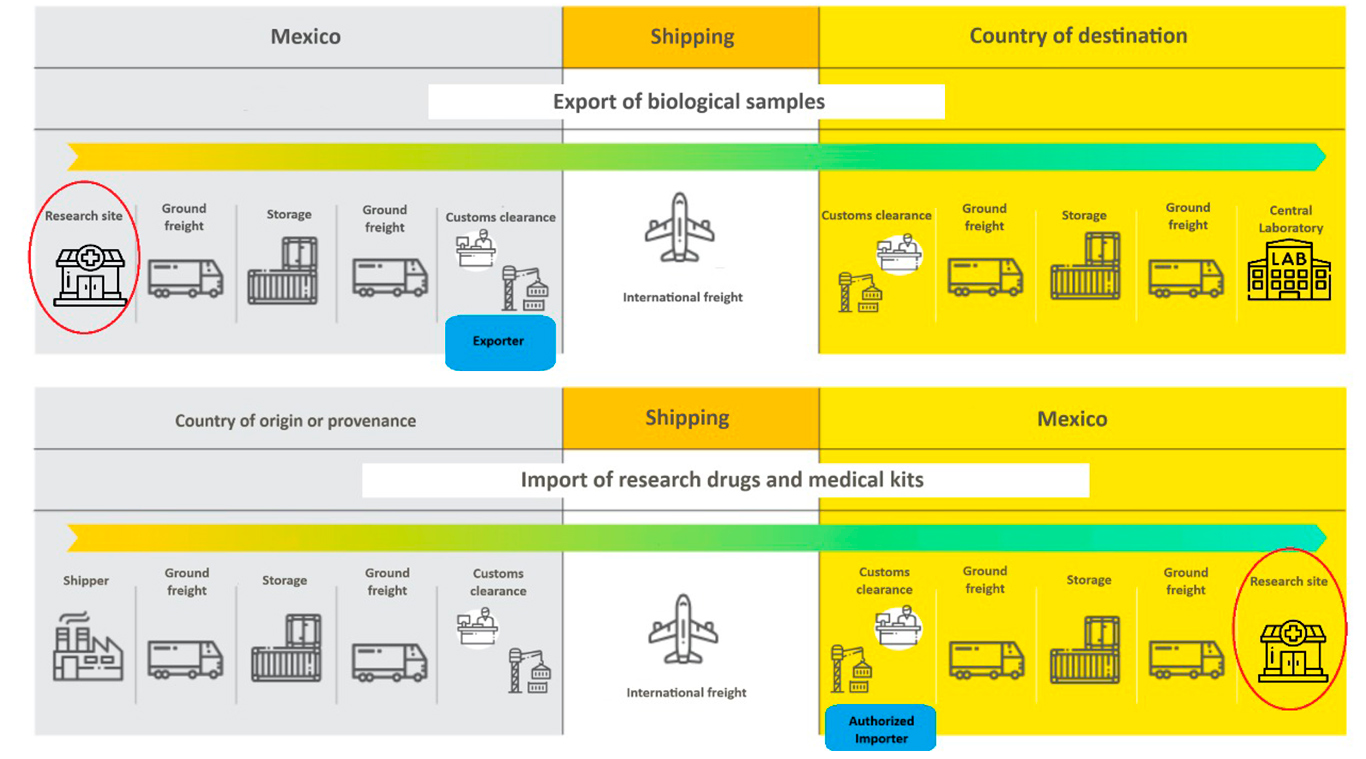
Flow of Materials to Mexico (Importation)
Medical kits (vacutainers, tubes, vials) and investigational drugs arrive from the U.S. or other countries to clinical sites in Mexico. Sanitary permits are required for customs clearance.
Permits Required for Importing Investigational Drugs.
When a drug enters Mexico exclusively for use in an authorized research protocol, sanitary registration is NOT required (unlike for commercial drugs). However, a specific import permit is mandatory.
The required permits are:
1. Protocol authorization issued by COFEPRIS
2. Import permit for investigational drugs
Permits Required for Importing Medical Kits (Auxiliary Study Materials)
These include essential items for sample collection or processing: tubes, swabs, lancets, collection containers, thermal envelopes, etc.
Required permits:
1. COFEPRIS import permit (if applicable): Required when kits contain materials intended for medical use. If the kits do not involve direct body contact or pose no health risk, they may be imported as supplies without sanitary registration, or under Rule 3.13 of the General Foreign Trade Rules (RGCE), if conditions are met.
2. Technical justification letter from the sponsor or CRO: A document stating that the kits have no commercial value, will be used solely for an authorized clinical protocol, and will not be sold or distributed.
Additional Considerations
Labeling:
• Medications must be labeled as "Investigational Medicinal Product – Exclusive use for clinical trials."
• Kits containing biological or hazardous materials require both internal and external labeling.
Transportation:
• Cold chain requirements must be demonstrated by proper packaging.
• Use of dry ice (UN 1845) must be declared as Class 9 dangerous goods.
Flow of Materials from Mexico (Export)
• Biological samples (blood, plasma, urine, tissue) collected at research sites are exported to central laboratories.
These samples are considered potentially hazardous biological materials regulated under UN 3373 – Biological Substance, Category B.
• The typical transit time from the clinical site to the USA is approximately 24 hours.
